Funded by the National Natural Science Foundation of China (Grant No. 21776024, 21761162015), the team of Wei Zidong / Ding Wei of Chongqing University has developed a ruthenium-based low-temperature fuel cell catalyst that can achieve highly selective hydrogenation under the coexistence of high concentration carbon monoxide. The research results, entitled "Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction", were published online in Nature Catalysis on April 27, 2020.
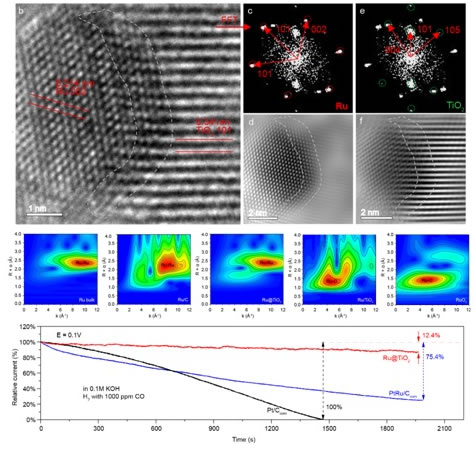
Figure 1. Lattice confined Ru cluster catalyst and its coordination structure and electrochemical properties
As a clean secondary energy source, the efficient use of hydrogen energy is an important chemical technology approach that couples traditional fossil energy and renewable energy systems to solve the problems of storage, transmission and distribution, and terminal applications of existing energy systems. Hydrogenation and its reverse reaction, hydrogen reduction, will inevitably become an important reaction dominating the hydrogen economy. At present, precious metal Pt is the most efficient and widely used hydrogenation catalyst at low pH. However, in the catalytic process, even if the reaction gas contains very little CO (10 ppm), the Pt-based catalyst will be completely poisoned and deactivated, making it impossible to use cheap and readily available fuel gas such as reformed gas, which greatly boosts the hydrogen. The technical difficulty of obtaining, purifying, storing and transporting gas in the economic and technological chain. In contrast, the price of metal Ru is only one third of Pt, but the scientific community widely believes that the surface of metal ruthenium (Ru) is easy to oxidize, even at a lower potential (0.1 ~ 0.3 V), the surface of metal Ru It will preferentially form the surface of Ru-Oads, which is not suitable as the main catalyst for hydrogenation.
Based on the mesoscale academic thought, this research work proposes a "lattice confinement type" Ru @ TiO2 catalyst with metal clusters embedded in oxide (as shown in Figure 1). Under a reducing atmosphere, during the crystallization process of amorphous TiO2, Ru nanoparticles grow clusters along the Ti atoms in the TiO2 lattice at the defect site to form a Ru @ TiO2 lattice structure connected by Ru-Ti metal bonds. This metal-bound lattice confinement structure leads to Ru in the interface. On the one hand, it has an atomic arrangement structure similar to TiO2 oxide (arranged according to TiO2 (101)), while maintaining its inherent metallicity, showing both Different from the Ru bulk metal, it is also different from the mesoscale behavior of Ru nanoclusters. For example, it has both the metallicity of the bulk and the low coordination of the cluster (CN = 7 ~ 8); it has the high stability as the bulk metal and the reactivity of the cluster metal; it has a greater than the metal oxygen bond (Ti- O, 0.193 nm) is lower than the Ru-Ti bond length (0.258 nm) of the metal bond (Ru-Ru, 0.267 nm). The metal bond at the Ru-Ti interface can promote the transfer of electrons from the electron-rich n-type semiconductor TiO2 to the d-band in the Ru cluster, so that the d-band of the Ru cluster is nearly completely filled. This d-band with almost full electrons is conducive to maintaining the metallic properties of Ru and improving its catalytic hydrogenation reaction activity. At the same time, it greatly reduces the probability of CO σ electrons transferring to the empty orbit of Ru metal d, weakening the strength of CO adsorption At the same time, it also leads to an increase in the concentration of O holes in TiO2, which is beneficial to the oxidative removal of CO, thus giving the catalyst excellent anti-CO poisoning properties. In the presence of 1000 ppm CO, the catalytic activity of hydrogen oxidation is almost undisturbed; even if the CO content is increased to ~ 10 vol%, the catalyst still selectively catalyzes the oxidation of hydrogen. In addition, on the surface of Ru clusters with almost full d-band electrons, Ru tends to be more stable and not easy to oxidize, so that the catalyst still maintains reactivity over a wide potential range. In acidic and alkaline media, the HOR mass activity (@ 0.02V) of Ru @ TiO2 catalyst is 30% to 15% higher than that of commercial PtRu / C catalyst; its super anti-oxidation is at a potential up to 0.9V ( Generally, metals are mainly produced on the surface by oxide species) Ru @ TiO2 catalyst Ru clusters still maintain the metallicity and catalytic hydrogenation characteristics of the surface.
In this study, a new type of electrocatalyst with high selectivity to oxidization against CO poisoning and surface oxidation was prepared by lattice-modulating the lattice and electronic structure of the metal in the interface region. The discovery breaks through the limit of low-temperature CO poisoning of precious metal catalysts, opens up new ideas for the use of semiconductor free electrons to regulate metal electronic structures, and lays the foundation for the efficient coupling of traditional fossil chemical technology and new energy chemical technology.
Komatsu Electrical Parts,Komatsu Forklift Parts,Excavator Electrical Parts,Quality Komatsu Electrical Parts
JINING SHANTE SONGZHENG CONSTRUCTION MACHINERY CO.LTD , https://www.sdkomatsudozerparts.com