 |
Toshiba has trial-produced an "artificial photosynthesis" system that mimics the photosynthesis of plants. It can use water (H2O), carbon dioxide (CO2O) and solar energy to make organic matter. The conversion efficiency obtained by dividing the incident light energy by the generated carbon monoxide (CO) energy reached 1.5%. According to reports, this value is comparable to the photosynthetic efficiency of algae in plants. For example, the energy conversion efficiency of Chlorella grown in aquaculture environment is about 2% Note 1).
Note 1) Calculated from the cellulose and carbohydrate conversion section.
Artificial photosynthesis can not only reduce the greenhouse effect of carbon dioxide, but also obtain organic materials used as energy and materials. Japan and other countries are promoting relevant research projects.
For example, in the NEDO project "Development of carbon dioxide feedstock basic chemical manufacturing process technology" that is expected to invest 11.6 billion yen in 2014-2021, research on artificial photosynthesis was carried out. The United States has also advanced research by investing US$122 million in the “Joint Center for Artificial Photosynthesis†scheduled for 2010–2015.
However, this result has nothing to do with the above projects, but Toshiba "spontaneous research."
Gold Nanocatalyst contributes to increase efficiency
Artificial photosynthesis first uses light energy to decompose water into protons (H+) and electrons (e-) (oxidation reactions). Then, protons and electrons are used to produce carbon monoxide, formic acid (HCOOH), and methane (CH4) from carbon dioxide (reduction reaction).
The reason why carbon monoxide can be generated at a high conversion efficiency this time is due to the use of a gold (Au) catalyst (Au nanocatalyst, Fig. 1) having a nano-sized structure on the reduction side. The use of Au nanocatalysts reduces the "overpressure" of the redox reaction (Figure 2). Overpressure refers to the difference between the "theoretical potential" of a substance in a stable state and the potential when it actually reacts.
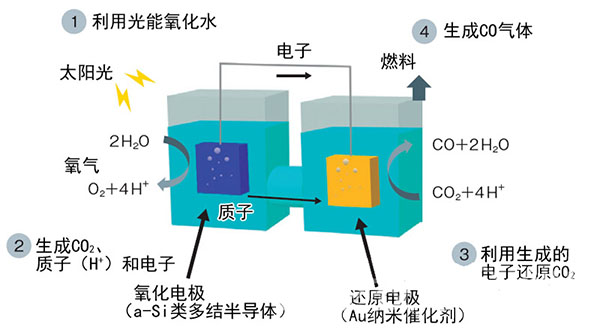
Figure 1: Using a-Si based semiconductors and Au nanocatalysts
In the Toshiba trial-manufactured artificial synthesis system, the oxidation electrode adopts a-Si type multi junction semiconductor. The reduction side uses an Au catalyst with a nano-sized structure. (The figure was produced by "Nikkei Electronics" based on Toshiba data)
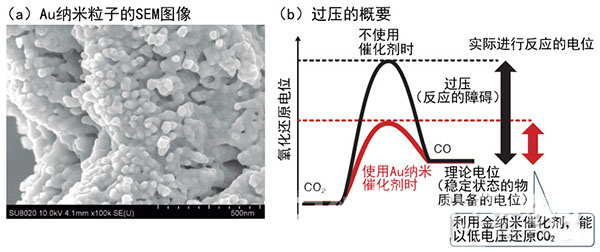
Figure 2: Use of Au Nanocatalyst to Promote Reaction
When reducing carbon monoxide with carbon dioxide, there is an overpressure that inhibits the redox reaction, and a higher potential than the theoretical potential is required. Toshiba uses Au nanoparticles as a catalyst on the reduction electrode side, reducing this obstacle. (Photograph: Toshiba, Figure (b) by "Nikkei Electronics" based on Toshiba data)
It is known that Au can excite the activity of the reduction reaction, but if the shape is a plate shape or the like, the efficiency does not increase. By making a nano-sized structure, high efficiency is achieved for the first time. Toshiba did not disclose the specific manufacturing method of the structure, but only said that "the use of high-frequency electrochemical methods."
Oxidation electrode low cost
By reducing the overvoltage, the oxidation electrode can use low-priced materials. Specifically, a material in which three-layer amorphous silicon (a-Si) based semiconductors having different absorption wavelengths are stacked is used ( FIG. 3 ). By connecting these layers in series, a voltage of 2.2 V required for the reduction reaction was obtained. Without the use of Au nanocatalysts, it is difficult to obtain carbon monoxide with a voltage of 2.2V due to the high overvoltage. Toshiba believes that a-Si-based semiconductors are "common materials used in solar cells and the like, and they should be able to reduce costs compared with oxidized electrodes using large bandgap compound semiconductors such as GaN."
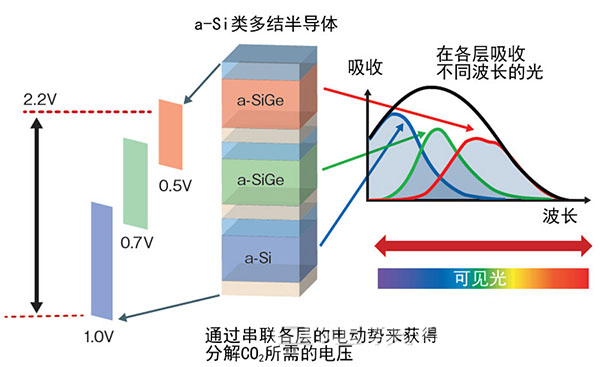
Figure 3: Stacking 3-layer semiconductors to increase voltage
The oxidation electrode uses a material in which three layers of a-Si semiconductors are stacked. Absorb different wavelengths of light in each layer. By connecting these layers in series, the potential required for the reduction reaction can be obtained. (The figure was produced by "Nikkei Electronics" based on Toshiba data)
By using an amorphous silicon-based semiconductor as an oxidizing electrode, light in the visible light region wavelength (380n to 780nm) can be utilized in artificial photosynthesis, thus contributing to improvement of efficiency. Because in the energy of solar energy, the visible light area accounts for more than half of the energy.
To increase the generated voltage, a large bandgap semiconductor such as GaN can also be used, but only short-wavelength light such as ultraviolet light can be used. In the energy of solar energy, the proportion of energy in the ultraviolet region is only about 3%.
Although the oxidizing electrode can reduce the cost, the cost is still relatively high because Au is used on the reduction side. This is one of the practical issues. As a countermeasure, it is conceivable to realize nanostructures of metals other than Au, or to reduce the amount of Au.
In fact, the mechanism by which Au nanocatalysts promote artificial photosynthesis reactions is "not very clear" (Toshiba). Therefore, by clarifying the reaction mechanism, it is expected to reduce the amount of Au. (Reporter: Neejin, "Nikkei Electronics")
Floor Lamp,Floor light, Standing Lamp,Led Floor Lamp
JINGYING , https://www.jingyingoptical.com