Energy is the material basis for the progress and development of human civilization. In recent years, with the gradual consumption of fossil energy and the increasingly prominent environmental pollution problems, human demand for green, clean and renewable energy has increased dramatically. The development of high-efficiency, low-cost energy storage and conversion technologies such as water splitting, fuel cells, and metal-air batteries has become the frontier of research. Among them, the use of aqueous electrolytes in zinc-air batteries has the advantages of low cost, safety, and environmental friendliness. The theoretical energy is as high as 1084 Wh / kg, which is expected to become a new generation of energy storage equipment. According to the needs of use, zinc-air batteries can be made into primary batteries, rechargeable batteries and flexible batteries. The discharge process of a zinc-air battery involves an oxygen reduction reaction (ORR), while the charging process involves an oxygen evolution reaction (OER). At present, Pt-based catalysts are excellent ORR catalysts. Ir and Ru-based catalysts have excellent catalytic performance in OER reactions, but platinum group elements have scarce reserves in the crust, are expensive, have poor stability, and have a single function. Therefore, the development of low-cost, high-efficiency, and stable non-precious metal catalysts is of great significance for the commercialization of zinc-air batteries.
Monoatomic catalysts have high intrinsic activity, maximized atom utilization efficiency, and specific catalyst structures. In recent years, the preparation, characterization and catalytic performance studies based on single-atom catalysts have become research hotspots in the fields of energy, materials and catalysis. Non-precious metal Fe-based, Co-based, Ni-based, and Mn-based single-atom catalysts show excellent electrocatalytic performance, and are expected to be a substitute for platinum-group precious metal catalysts. In particular, Fe-based single-atom catalysts have better performance than Pt-based catalysts in ORR reaction under alkaline conditions, showing higher half-wave potential, greater limiting current density and diffusion current density.
Recently, Han Junxing, associate researcher of Sun Chunwen's research group at the Beijing Institute of Nano-Energy and Systems, Chinese Academy of Sciences, and others successfully prepared monoatomic Fe-based catalysts based on metal-organic framework materials (MOF) coating and high-temperature cracking technology. In this work, divalent FeSO4 was used as the precursor of Fe; 1,10-o-phenanthroline was used as the organic ligand (Phen) to form an organic complex (Fe-Phen) through coordination with Fe2 + ions. During the growth of MOF crystals, organic compound molecules (Fe-Phen) are encapsulated in situ in a nano-cavity with molecular size, separated from each other by the MOF framework. A single atom-dispersed Fe-based catalyst was obtained after roasting at 900 ° C under Ar atmosphere. The electrochemical test results show that the half-wave potential of the single-atom Fe-based catalyst in the ORR reaction is as high as 0.91 V, which is 90 mV higher than that of the traditional Pt / C catalyst, and is superior to most of the catalysts reported in the current literature; About twice the commercial Pt / C catalyst. The single-atom Fe-based catalyst is used as the positive electrode catalyst for primary zinc-air batteries. The open circuit voltage of the battery is up to 1.51 V, which is better than Pt / C catalyst (1.45 V); the power density reaches 96.4 mW cm-2; When the current density is discharged, the primary zinc-air battery can be stably operated for more than 2000 min at a discharge voltage of 1.28 V.
In addition to exhibiting excellent ORR catalytic performance, single-atom Fe-based catalysts also have better bifunctional (ORR / OER) catalytic properties. The potential difference between Ej10 and E1 / 2 is 0.92 V, which is smaller than that of precious metal composite catalysts (Pt / C + RuO2) (0.94 V). The single-atom Fe-based catalyst is used as the positive electrode of a rechargeable zinc-air battery. The zinc-air battery can operate stably for more than 250 h at a current density of 10 mA cm-2. The single-atom Fe catalyst is used as the positive electrode of a flexible foldable zinc-air battery, and the battery can operate stably for more than 120 h at a current density of 1 mA cm-2.
Related research results are published in the latest issue of "Advanced Functional Materials" under the title of Single-Atom Fe-Nx-C as an Efficient Electrocatalyst for Zinc-Air Batteries. This work was supported by the National Key R & D Program of the Ministry of Science and Technology (2016YFA0202702) and the National Natural Science Foundation of China (51672029, 51372271).
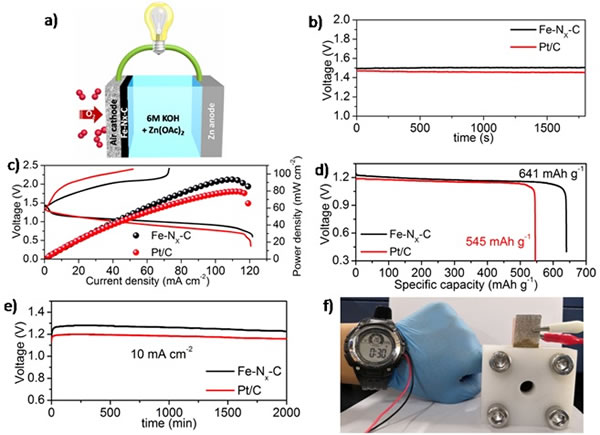
Figure 1. a) Schematic diagram of primary zinc-air battery structure; b) open circuit voltage variation curve of battery prepared with different catalysts over time; c) battery power density characteristics; d) battery discharge curve; e) at 10 mA cm-2 The constant current discharge curve of the battery under the current density; f) Show that the zinc-air battery can power the electronic watch.
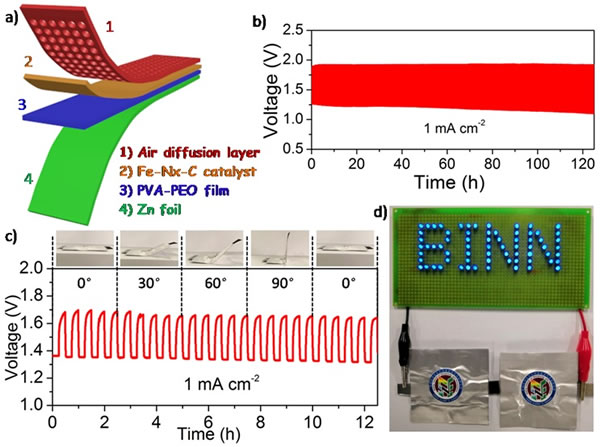
Figure 2. a) Schematic diagram of a flexible foldable zinc-air battery; b) Battery charge and discharge curve at a current density of 1 mA cm-2; c) Folding performance test of a flexible zinc-air battery; d) Two sets of flexible in series The zinc-air battery powers the LED array.
Safety Valve,Square Breathing Valve,Pressure Regulating Valves,Manual Control Exhaust Valve
Ningbo Wenhan Fluid Equipments Co., Ltd. , https://www.wenhanvalves.com